Jump to navigation
Jump to search
NCBI: 03-AUG-2016
⊟Summary[edit | edit source]
- organism: Staphylococcus aureus NCTC8325
- locus tag: SAOUHSC_02511
- pan locus tag?: SAUPAN005701000
- symbol: rplD
- pan gene symbol?: rplD
- synonym:
- product: 50S ribosomal protein L4
⊟Genome View[edit | edit source]
⊟Gene[edit | edit source]
⊟General[edit | edit source]
⊟Accession numbers[edit | edit source]
- Gene ID: 3920887 NCBI
- RefSeq: YP_500978 NCBI
- BioCyc: G1I0R-2374 BioCyc
- MicrobesOnline: 1290949 MicrobesOnline
⊟Phenotype[edit | edit source]
Share your knowledge and add information here. [edit]
⊟DNA sequence[edit | edit source]
- 1
61
121
181
241
301
361
421
481
541
601ATGGCTAATTATGATGTTTTAAAATTAGACGGAACTAAATCAGGTTCAATCGAATTAAGC
GATGCAGTATTCGGTATTGAGCCAAATAATAGCGTTTTATTCGAAGCTATTAATTTACAA
CGTGCTTCATTACGTCAAGGTACGCATGCTGTTAAGAATCGTTCAGCAGTAAGCGGTGGC
GGACGTAAACCATGGAAGCAAAAAGGAACAGGTCGTGCTCGTCAAGGTACAATCCGTGCT
CCACAATGGCGTGGCGGTGGTATCGTATTCGGACCAACTCCAAGAAGTTATGCATACAAA
ATGCCTAAGAAAATGCGTCGTTTAGCTTTACGCTCAGCATTATCTTTCAAAGCTCAAGAG
AATGGCTTAACTGTAGTTGACGCATTCAACTTCGAAGCTCCAAAAACTAAAGAATTCAAA
AATGTATTATCTACATTAGAACAACCTAAAAAAGTATTAGTAGTTACTGAAAACGAAGAT
GTAAATGTTGAATTATCAGCACGCAACATCCCTGGCGTTCAAGTGACAACTGCTCAAGGT
TTAAATGTTTTAGATATCACTAATGCTGACAGCTTAGTAATTACTGAAGCTGCTGCTAAA
AAAGTTGAGGAGGTGCTCGGATAA60
120
180
240
300
360
420
480
540
600
624
⊟Protein[edit | edit source]
Protein Data Bank: 4WCE
Protein Data Bank: 4WF9
Protein Data Bank: 4WFA
Protein Data Bank: 4WFB
Protein Data Bank: 5HKV
Protein Data Bank: 5HL7
Protein Data Bank: 5LI0
Protein Data Bank: 5ND8
Protein Data Bank: 5ND9
Protein Data Bank: 5NRG
Protein Data Bank: 5TCU
⊟General[edit | edit source]
- locus tag: SAOUHSC_02511
- symbol: RplD
- description: 50S ribosomal protein L4
- length: 207
- theoretical pI: 10.5993
- theoretical MW: 22464.5
- GRAVY: -0.348309
⊟Function[edit | edit source]
- TIGRFAM: Protein synthesis Ribosomal proteins: synthesis and modification 50S ribosomal protein uL4 (TIGR03953; HMM-score: 272.5)and 1 more50S ribosomal protein uL4 (TIGR03672; HMM-score: 38.3)
- TheSEED :
- LSU ribosomal protein L4p (L1e)
- PFAM: no clan defined Ribosomal_L4; Ribosomal protein L4/L1 family (PF00573; HMM-score: 260.5)and 1 moreConcanavalin (CL0004) Reoviridae_Vp9; Reoviridae VP9 (PF08978; HMM-score: 12)
⊟Structure, modifications & cofactors[edit | edit source]
- domains:
- modifications:
- cofactors:
- effectors:
⊟Localization[edit | edit source]
- PSORTb: unknown (no significant prediction)
- Cytoplasmic Score: 2.5
- Cytoplasmic Membrane Score: 2.5
- Cellwall Score: 2.5
- Extracellular Score: 2.5
- Internal Helices: 0
- DeepLocPro: Extracellular
- Cytoplasmic Score: 0.3165
- Cytoplasmic Membrane Score: 0.0016
- Cell wall & surface Score: 0.0002
- Extracellular Score: 0.6817
- LocateP: Intracellular
- Prediction by SwissProt Classification: Cytoplasmic
- Pathway Prediction: No pathway
- Intracellular possibility: 1
- Signal peptide possibility: -1
- N-terminally Anchored Score: -1
- Predicted Cleavage Site: No CleavageSite
- SignalP: no predicted signal peptide
- SP(Sec/SPI): 0.005565
- TAT(Tat/SPI): 0.002315
- LIPO(Sec/SPII): 0.000737
- predicted transmembrane helices (TMHMM): 0
⊟Accession numbers[edit | edit source]
⊟Protein sequence[edit | edit source]
- MANYDVLKLDGTKSGSIELSDAVFGIEPNNSVLFEAINLQRASLRQGTHAVKNRSAVSGGGRKPWKQKGTGRARQGTIRAPQWRGGGIVFGPTPRSYAYKMPKKMRRLALRSALSFKAQENGLTVVDAFNFEAPKTKEFKNVLSTLEQPKKVLVVTENEDVNVELSARNIPGVQVTTAQGLNVLDITNADSLVITEAAAKKVEEVLG
⊟Experimental data[edit | edit source]
- experimentally validated: PeptideAtlas [2] [3]
- protein localization: data available for COL
- quantitative data / protein copy number per cell: data available for COL
- interaction partners:
SAOUHSC_01683 (dnaK) molecular chaperone DnaK [4] (data from MRSA252) SAOUHSC_00799 (eno) phosphopyruvate hydratase [4] (data from MRSA252) SAOUHSC_00574 (eutD) phosphotransacetylase [4] (data from MRSA252) SAOUHSC_02116 (gatB) aspartyl/glutamyl-tRNA amidotransferase subunit B [4] (data from MRSA252) SAOUHSC_02254 (groEL) chaperonin GroEL [4] (data from MRSA252) SAOUHSC_02255 (groES) co-chaperonin GroES [4] (data from MRSA252) SAOUHSC_01246 (infB) translation initiation factor IF-2 [4] (data from MRSA252) SAOUHSC_01786 (infC) translation initiation factor IF-3 [4] (data from MRSA252) SAOUHSC_00900 (pgi) glucose-6-phosphate isomerase [4] (data from MRSA252) SAOUHSC_00796 (pgk) phosphoglycerate kinase [4] (data from MRSA252) SAOUHSC_02368 (pyrG) CTP synthetase [4] (data from MRSA252) SAOUHSC_00519 (rplA) 50S ribosomal protein L1 [4] (data from MRSA252) SAOUHSC_02509 (rplB) 50S ribosomal protein L2 [4] (data from MRSA252) SAOUHSC_02512 (rplC) 50S ribosomal protein L3 [4] (data from MRSA252) SAOUHSC_02500 (rplE) 50S ribosomal protein L5 [4] (data from MRSA252) SAOUHSC_02496 (rplF) 50S ribosomal protein L6 [4] (data from MRSA252) SAOUHSC_00017 (rplI) 50S ribosomal protein L9 [4] (data from MRSA252) SAOUHSC_00520 (rplJ) 50S ribosomal protein L10 [4] (data from MRSA252) SAOUHSC_02478 (rplM) 50S ribosomal protein L13 [4] (data from MRSA252) SAOUHSC_02502 (rplN) 50S ribosomal protein L14 [4] (data from MRSA252) SAOUHSC_02492 (rplO) 50S ribosomal protein L15 [4] (data from MRSA252) SAOUHSC_02495 (rplR) 50S ribosomal protein L18 [4] (data from MRSA252) SAOUHSC_01211 (rplS) 50S ribosomal protein L19 [4] (data from MRSA252) SAOUHSC_01784 (rplT) 50S ribosomal protein L20 [4] (data from MRSA252) SAOUHSC_01757 (rplU) 50S ribosomal protein L21 [4] (data from MRSA252) SAOUHSC_02507 (rplV) 50S ribosomal protein L22 [4] (data from MRSA252) SAOUHSC_02510 (rplW) 50S ribosomal protein L23 [4] (data from MRSA252) SAOUHSC_02501 (rplX) 50S ribosomal protein L24 [4] (data from MRSA252) SAOUHSC_01755 (rpmA) 50S ribosomal protein L27 [4] (data from MRSA252) SAOUHSC_02493 (rpmD) 50S ribosomal protein L30 [4] (data from MRSA252) SAOUHSC_01785 (rpmI) 50S ribosomal protein L35 [4] (data from MRSA252) SAOUHSC_01493 (rpsA) 30S ribosomal protein S1 [4] (data from MRSA252) SAOUHSC_01232 (rpsB) 30S ribosomal protein S2 [4] (data from MRSA252) SAOUHSC_02506 (rpsC) 30S ribosomal protein S3 [4] (data from MRSA252) SAOUHSC_01829 (rpsD) 30S ribosomal protein S4 [4] (data from MRSA252) SAOUHSC_02494 (rpsE) 30S ribosomal protein S5 [4] (data from MRSA252) SAOUHSC_00348 (rpsF) 30S ribosomal protein S6 [4] (data from MRSA252) SAOUHSC_02498 (rpsH) 30S ribosomal protein S8 [4] (data from MRSA252) SAOUHSC_02477 (rpsI) 30S ribosomal protein S9 [4] (data from MRSA252) SAOUHSC_00527 (rpsL) 30S ribosomal protein S12 [4] (data from MRSA252) SAOUHSC_02487 (rpsM) 30S ribosomal protein S13 [4] (data from MRSA252) SAOUHSC_02499 (rpsN) 30S ribosomal protein S14 [4] (data from MRSA252) SAOUHSC_01208 (rpsP) 30S ribosomal protein S16 [4] (data from MRSA252) SAOUHSC_02503 (rpsQ) 30S ribosomal protein S17 [4] (data from MRSA252) SAOUHSC_02508 (rpsS) 30S ribosomal protein S19 [4] (data from MRSA252) SAOUHSC_01689 (rpsT) 30S ribosomal protein S20 [4] (data from MRSA252) SAOUHSC_01418 (sucA) 2-oxoglutarate dehydrogenase E1 component [4] (data from MRSA252) SAOUHSC_01216 (sucC) succinyl-CoA synthetase subunit beta [4] (data from MRSA252) SAOUHSC_01788 (thrS) threonyl-tRNA synthetase [4] (data from MRSA252) SAOUHSC_01779 (tig) trigger factor [4] (data from MRSA252) SAOUHSC_00797 (tpiA) triosephosphate isomerase [4] (data from MRSA252) SAOUHSC_01234 (tsf) elongation factor Ts [4] (data from MRSA252) SAOUHSC_00069 protein A [4] (data from MRSA252) SAOUHSC_00187 formate acetyltransferase [4] (data from MRSA252) SAOUHSC_00206 L-lactate dehydrogenase [4] (data from MRSA252) SAOUHSC_00365 alkyl hydroperoxide reductase subunit C [4] (data from MRSA252) SAOUHSC_00444 hypothetical protein [4] (data from MRSA252) SAOUHSC_00461 methionyl-tRNA synthetase [4] (data from MRSA252) SAOUHSC_00474 50S ribosomal protein L25/general stress protein Ctc [4] (data from MRSA252) SAOUHSC_00483 hypothetical protein [4] (data from MRSA252) SAOUHSC_00488 hypothetical protein [4] (data from MRSA252) SAOUHSC_00499 pyridoxal biosynthesis lyase PdxS [4] (data from MRSA252) SAOUHSC_00525 DNA-directed RNA polymerase subunit beta' [4] (data from MRSA252) SAOUHSC_00528 30S ribosomal protein S7 [4] (data from MRSA252) SAOUHSC_00529 elongation factor G [4] (data from MRSA252) SAOUHSC_00530 elongation factor Tu [4] (data from MRSA252) SAOUHSC_00562 phosphomethylpyrimidine kinase [4] (data from MRSA252) SAOUHSC_00573 heme peroxidase [4] (data from MRSA252) SAOUHSC_00608 alcohol dehydrogenase [4] (data from MRSA252) SAOUHSC_00694 hypothetical protein [4] (data from MRSA252) SAOUHSC_00767 hypothetical protein [4] (data from MRSA252) SAOUHSC_00785 thioredoxin reductase [4] (data from MRSA252) SAOUHSC_00795 glyceraldehyde-3-phosphate dehydrogenase [4] (data from MRSA252) SAOUHSC_00798 2,3-bisphosphoglycerate-independent phosphoglycerate mutase [4] (data from MRSA252) SAOUHSC_00835 hypothetical protein [4] (data from MRSA252) SAOUHSC_00836 glycine cleavage system protein H [4] (data from MRSA252) SAOUHSC_00891 cyclophilin-type peptidyl-prolyl cis-trans isomerase [4] (data from MRSA252) SAOUHSC_00906 hypothetical protein [4] (data from MRSA252) SAOUHSC_00908 coenzyme A disulfide reductase [4] (data from MRSA252) SAOUHSC_00937 oligoendopeptidase F [4] (data from MRSA252) SAOUHSC_01007 bifunctional 5,10-methylene-tetrahydrofolate dehydrogenase/ 5,10-methylene-tetrahydrofolate cyclohydrolase [4] (data from MRSA252) SAOUHSC_01028 phosphocarrier protein HPr [4] (data from MRSA252) SAOUHSC_01029 phosphoenolpyruvate-protein phosphotransferase [4] (data from MRSA252) SAOUHSC_01036 hypothetical protein [4] (data from MRSA252) SAOUHSC_01040 pyruvate dehydrogenase complex, E1 component subunit alpha [4] (data from MRSA252) SAOUHSC_01041 pyruvate dehydrogenase complex, E1 component subunit beta [4] (data from MRSA252) SAOUHSC_01042 branched-chain alpha-keto acid dehydrogenase subunit E2 [4] (data from MRSA252) SAOUHSC_01043 dihydrolipoamide dehydrogenase [4] (data from MRSA252) SAOUHSC_01100 thioredoxin [4] (data from MRSA252) SAOUHSC_01149 cell division protein [4] (data from MRSA252) SAOUHSC_01150 cell division protein FtsZ [4] (data from MRSA252) SAOUHSC_01199 3-oxoacyl-(acyl-carrier-protein) reductase [4] (data from MRSA252) SAOUHSC_01218 succinyl-CoA synthetase subunit alpha [4] (data from MRSA252) SAOUHSC_01251 polynucleotide phosphorylase/polyadenylase [4] (data from MRSA252) SAOUHSC_01287 glutamine synthetase [4] (data from MRSA252) SAOUHSC_01337 transketolase [4] (data from MRSA252) SAOUHSC_01416 dihydrolipoamide succinyltransferase [4] (data from MRSA252) SAOUHSC_01490 DNA-binding protein HU [4] (data from MRSA252) SAOUHSC_01605 6-phosphogluconate dehydrogenase [4] (data from MRSA252) SAOUHSC_01625 elongation factor P [4] (data from MRSA252) SAOUHSC_01653 superoxide dismutase [4] (data from MRSA252) SAOUHSC_01666 glycyl-tRNA synthetase [4] (data from MRSA252) SAOUHSC_01801 isocitrate dehydrogenase [4] (data from MRSA252) SAOUHSC_01806 pyruvate kinase [4] (data from MRSA252) SAOUHSC_01807 6-phosphofructokinase [4] (data from MRSA252) SAOUHSC_01814 hypothetical protein [4] (data from MRSA252) SAOUHSC_01820 acetate kinase [4] (data from MRSA252) SAOUHSC_01867 D-alanine aminotransferase [4] (data from MRSA252) SAOUHSC_01901 putative translaldolase [4] (data from MRSA252) SAOUHSC_01987 hypothetical protein [4] (data from MRSA252) SAOUHSC_02108 ferritin [4] (data from MRSA252) SAOUHSC_02140 manganese-dependent inorganic pyrophosphatase [4] (data from MRSA252) SAOUHSC_02150 hypothetical protein [4] (data from MRSA252) SAOUHSC_02365 UDP-N-acetylglucosamine 1-carboxyvinyltransferase [4] (data from MRSA252) SAOUHSC_02366 fructose-bisphosphate aldolase [4] (data from MRSA252) SAOUHSC_02377 pyrimidine-nucleoside phosphorylase [4] (data from MRSA252) SAOUHSC_02399 glucosamine--fructose-6-phosphate aminotransferase [4] (data from MRSA252) SAOUHSC_02425 hypothetical protein [4] (data from MRSA252) SAOUHSC_02441 alkaline shock protein 23 [4] (data from MRSA252) SAOUHSC_02486 30S ribosomal protein S11 [4] (data from MRSA252) SAOUHSC_02869 1-pyrroline-5-carboxylate dehydrogenase [4] (data from MRSA252) SAOUHSC_02922 L-lactate dehydrogenase [4] (data from MRSA252) SAOUHSC_02926 fructose-1,6-bisphosphate aldolase [4] (data from MRSA252) SAOUHSC_02927 malate:quinone oxidoreductase [4] (data from MRSA252) SAOUHSC_02968 ornithine carbamoyltransferase [4] (data from MRSA252) SAOUHSC_02969 arginine deiminase [4] (data from MRSA252)
⊟Expression & Regulation[edit | edit source]
⊟Operon[edit | edit source]
⊟Regulation[edit | edit source]
- regulator:
⊟Transcription pattern[edit | edit source]
- S.aureus Expression Data Browser: [5]
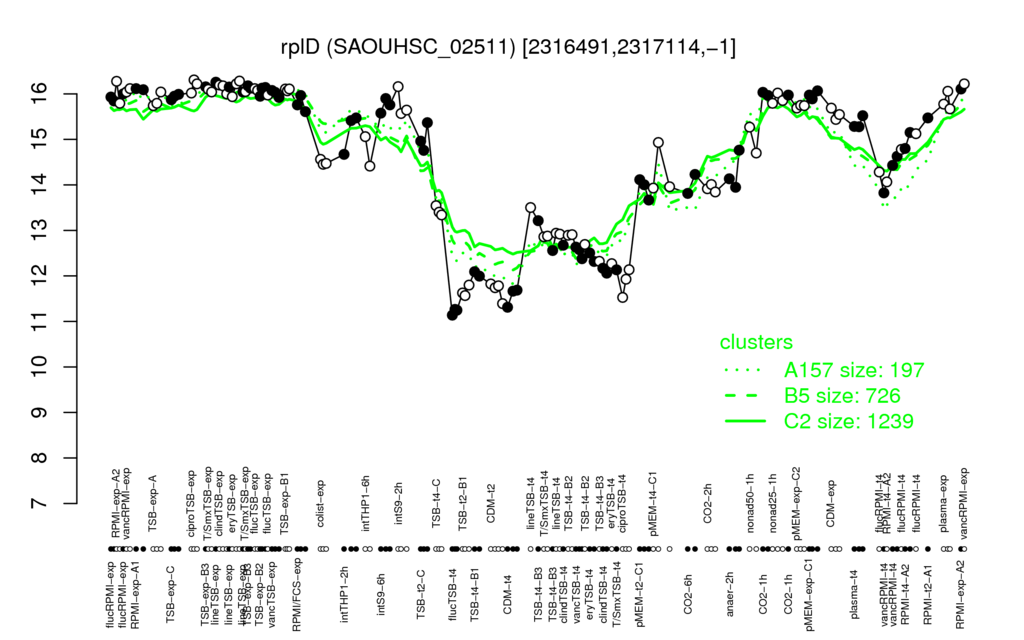 Multi-gene expression profiles
Multi-gene expression profiles
⊟Protein synthesis (provided by Aureolib)[edit | edit source]
⊟Protein stability[edit | edit source]
- half-life: no data available
⊟Biological Material[edit | edit source]
⊟Mutants[edit | edit source]
⊟Expression vector[edit | edit source]
⊟lacZ fusion[edit | edit source]
⊟GFP fusion[edit | edit source]
⊟two-hybrid system[edit | edit source]
⊟FLAG-tag construct[edit | edit source]
⊟Antibody[edit | edit source]
⊟Other Information[edit | edit source]
You can add further information about the gene and protein here. [edit]
⊟Literature[edit | edit source]
⊟References[edit | edit source]
- ↑ Roy R Chaudhuri, Andrew G Allen, Paul J Owen, Gil Shalom, Karl Stone, Marcus Harrison, Timothy A Burgis, Michael Lockyer, Jorge Garcia-Lara, Simon J Foster, Stephen J Pleasance, Sarah E Peters, Duncan J Maskell, Ian G Charles
Comprehensive identification of essential Staphylococcus aureus genes using Transposon-Mediated Differential Hybridisation (TMDH).
BMC Genomics: 2009, 10;291
[PubMed:19570206] [WorldCat.org] [DOI] (I e) - ↑ Maren Depke, Stephan Michalik, Alexander Rabe, Kristin Surmann, Lars Brinkmann, Nico Jehmlich, Jörg Bernhardt, Michael Hecker, Bernd Wollscheid, Zhi Sun, Robert L Moritz, Uwe Völker, Frank Schmidt
A peptide resource for the analysis of Staphylococcus aureus in host-pathogen interaction studies.
Proteomics: 2015, 15(21);3648-61
[PubMed:26224020] [WorldCat.org] [DOI] (I p) - ↑ Stephan Michalik, Maren Depke, Annette Murr, Manuela Gesell Salazar, Ulrike Kusebauch, Zhi Sun, Tanja C Meyer, Kristin Surmann, Henrike Pförtner, Petra Hildebrandt, Stefan Weiss, Laura Marcela Palma Medina, Melanie Gutjahr, Elke Hammer, Dörte Becher, Thomas Pribyl, Sven Hammerschmidt, Eric W Deutsch, Samuel L Bader, Michael Hecker, Robert L Moritz, Ulrike Mäder, Uwe Völker, Frank Schmidt
A global Staphylococcus aureus proteome resource applied to the in vivo characterization of host-pathogen interactions.
Sci Rep: 2017, 7(1);9718
[PubMed:28887440] [WorldCat.org] [DOI] (I e) - ↑ 4.000 4.001 4.002 4.003 4.004 4.005 4.006 4.007 4.008 4.009 4.010 4.011 4.012 4.013 4.014 4.015 4.016 4.017 4.018 4.019 4.020 4.021 4.022 4.023 4.024 4.025 4.026 4.027 4.028 4.029 4.030 4.031 4.032 4.033 4.034 4.035 4.036 4.037 4.038 4.039 4.040 4.041 4.042 4.043 4.044 4.045 4.046 4.047 4.048 4.049 4.050 4.051 4.052 4.053 4.054 4.055 4.056 4.057 4.058 4.059 4.060 4.061 4.062 4.063 4.064 4.065 4.066 4.067 4.068 4.069 4.070 4.071 4.072 4.073 4.074 4.075 4.076 4.077 4.078 4.079 4.080 4.081 4.082 4.083 4.084 4.085 4.086 4.087 4.088 4.089 4.090 4.091 4.092 4.093 4.094 4.095 4.096 4.097 4.098 4.099 4.100 4.101 4.102 4.103 4.104 4.105 4.106 4.107 4.108 4.109 4.110 4.111 4.112 4.113 4.114 4.115 4.116 4.117 4.118 4.119 4.120 4.121 4.122 4.123 4.124 4.125 Artem Cherkasov, Michael Hsing, Roya Zoraghi, Leonard J Foster, Raymond H See, Nikolay Stoynov, Jihong Jiang, Sukhbir Kaur, Tian Lian, Linda Jackson, Huansheng Gong, Rick Swayze, Emily Amandoron, Farhad Hormozdiari, Phuong Dao, Cenk Sahinalp, Osvaldo Santos-Filho, Peter Axerio-Cilies, Kendall Byler, William R McMaster, Robert C Brunham, B Brett Finlay, Neil E Reiner
Mapping the protein interaction network in methicillin-resistant Staphylococcus aureus.
J Proteome Res: 2011, 10(3);1139-50
[PubMed:21166474] [WorldCat.org] [DOI] (I p) - ↑ 5.0 5.1 Ulrike Mäder, Pierre Nicolas, Maren Depke, Jan Pané-Farré, Michel Debarbouille, Magdalena M van der Kooi-Pol, Cyprien Guérin, Sandra Dérozier, Aurelia Hiron, Hanne Jarmer, Aurélie Leduc, Stephan Michalik, Ewoud Reilman, Marc Schaffer, Frank Schmidt, Philippe Bessières, Philippe Noirot, Michael Hecker, Tarek Msadek, Uwe Völker, Jan Maarten van Dijl
Staphylococcus aureus Transcriptome Architecture: From Laboratory to Infection-Mimicking Conditions.
PLoS Genet: 2016, 12(4);e1005962
[PubMed:27035918] [WorldCat.org] [DOI] (I e)
